In September, several CCEPH investigators travelled to Northern Ireland to present research findings at the ‘Options for the Control of Influenza’ conference. This is the only global scientific meeting with a dedicated focus on influenza, occurring every 2-3 years. The Belfast conference was the 11th in the series, and CCEPH researchers have attended these meetings since 2007. The meeting addressed a wide range of issues related to influenza epidemiology, vaccines, basic science, and public health. It was a unique forum for exchange of ideas between government agencies, academia and industry. This was the first in-person Options meeting since the pandemic and it included an additional focus on COVID-19 epidemiology and vaccines.
The CCEPH research findings covered a broad range of topics including influenza vaccine effectiveness and COVID-19 booster effectiveness. Kayla Hanson, MPH gave on oral presentation on a study of cell-culture flu vaccine (Flucelvax, Seqirus) effectiveness in the 2019-20 season. In this study led by Huong McLean, PhD, patients with flu like illness are enrolled and swabs are collected for influenza testing. Vaccine effectiveness is estimated separately for the cell culture vaccine and standard egg-based vaccines. The CCEPH study found that flu vaccines were effective for preventing medical visits for confirmed influenza, and there was no significant difference between the cell culture vaccine and the egg-based vaccines. The study, which is funded by Seqirus, will continue in the 2022-23 flu season.
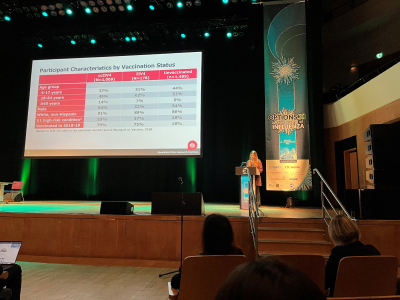
Jennifer King, MPH presented 2 CCEPH studies: a clinical trial of flu vaccine antibody response and an observational study COVID-19 booster dose effectiveness in a Marshfield area community cohort. The clinical trial compared the antibody response to 3 different flu vaccine products in working age adults over 2 flu seasons. Participants who received a recombinant vaccine (Flublok, Sanofi) that is manufactured without eggs had the best antibody response to the H3N2 flu viruses compared to inactivated egg-based and cell culture vaccines. The recombinant vaccine is one of three vaccines that are preferentially recommended for use in adults who are at least 65 years old. The other two recommended vaccines for older adults are Fluzone High Dose (Sanofi) and Fluad (Seqirus). Ms. King also presented results from an observational study of COVID-19 booster effectiveness. Huong McLean, PhD is the principal investigator. The Prospective Assessment of COVID-19 in a Community (PACC) study followed volunteers for over a year and collected nasal swabs to identify COVID-19 cases. Ms. King’s presentation highlighted the major finding that a booster dose of mRNA vaccine reduced the risk of infection with the Omicron variant by 48% compared to the 2-dose primary series.
Maria Sundaram, PhD presented results from a study of flu B vaccine effectiveness and the effect of prior vaccination. In this study, vaccine effectiveness was compared for two different lineages (strains) of influenza B among people who were vaccinated for 2 seasons in a row and those vaccinated in a single season. The findings suggested complex interactions between the vaccine lineage, circulating B viruses, and prior season vaccination. Overall, this study and others support the benefit of receiving a flu vaccine every year compared to remaining unvaccinated.
Joshua Petrie, PhD presented antibody test results from the PACC study. Study volunteers provided blood samples at different time points to look for antibodies generated by vaccination or infection. This is one of several planned analyses from this community cohort, and it showed that most people had antibodies to the SARS-CoV-2 virus by fall of 2021.